Background:The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection associated with coronavirus disease 2019 (COVID-19) causes a 3- to 9-fold higher age-adjusted mortality in African American and Hispanic populations, the major US racial groups affected by sickle cell disease (SCD). The Centers for Disease Control designates SCD as a condition at increased risk for severe COVID-19. An urgent need for repurposing of available and safe therapeutics has been cited for such high-risk populations until vaccines are widely available.
L-glutamine (GLN) ameliorates clinical pathology of SCD related to elements of COVID-19. Multiple systemic complications of COVID-19 are increasingly attributed to oxidative damage, a target which GLN regulates. Prescription-grade L-glutamine (PGLG) (Endari®, Emmaus Medical) decreases oxidative stress by increasing the ratio of reduced nicotinamide adenine dinucleotide (NAD) to total NAD, which may increase availability of reduced glutathione. PGLG also decreases red cell endothelial adhesion in patients with SCD. Of note, additional analysis of the phase 3 trial demonstrated a 63% lower occurrence of acute chest syndrome (ACS) in PGLG-treated SCD patients compared to control, which has important relevance in the pandemic. A recent report of two computational screens of FDA-approved therapeutics, directed to protein and chemistry targets and to gene expression changes induced by SARS-CoV-2, predicts glutathione and GLN are highly likely to confer benefit in COVID-19 (Kim, J Translat Med, 2020).
Methods:We therefore reviewed reports of multi-system effects of GLN in experimental respiratory distress animal models and in ICU and COVID-19 patients. We focused on contributors to cytokine storm and acute respiratory distress syndrome (ARDS), the leading causes of mortality in COVID-19 (Huang, Lancet, 2020). We also conducted a clinical trial in hospitalized COVID-19 patients on ESPEN-recommended nutrition +/- GLN.
Results:In experimental ARDS, sepsis, and endotoxin-induced lung injury, GLN decreases consolidation, pulmonary edema, and neutrophil infiltration and increases lung compliance, oxygen saturation, heat shock protein activation, and survival by 2.5-fold over saline controls (Perng WC, Clin Exp Pharmacol Physiol, 2010; Singleton, Crit Care Med, 2005). Patients with severe COVID-19 have increased proinflammatory cytokines; interleukin 6 (IL-6) levels predict and contribute to severity of COVID-19 (Yuki, Clin Immunol, 2020). GLN modulates inflammatory responses by suppressing C-reactive protein, IL-6, and TNF-α release; it also reduces IL-6 in murine studies (37% decrease,p< 0.05; Chuang, BMC Pulm Med, 2014), which could benefit COVID-19 patients.
Myocardial injury occurs in up to 12% of COVID-19 patients directly with viral entry through ACE-2 receptors, microvascular damage, endothelial shedding, and inflammation-mediated damage, which GLN protects against (Shi, Eur Heart J, 2020; Shi, JAMA Cardiol, 2020; Shao, Pak J Med Sci, 2015). Inflammatory states lead to GLN consumption and negative GLN balance (Santos, Amino Acids, 2019). Deficient plasma GLN (< 420 µmol/L) is a defined risk for higher mortality in ICU and COVID-19 patients (Shen, Cell, 2020). Glutathione deficiency contributes to SARS-CoV-2 oxidative lung damage and severe disease (Polonikov, ACS Infect Dis, 2020).
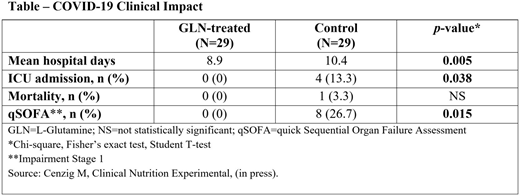
In a recent clinical trial, patients were confirmed to have SARS-CoV-2 by RT-PCR, had positive CT scans, and were admitted from a COVID-19 clinic. Both arms received ESPEN-recommended nutrition for COVID-19 alone or with GLN (10 grams, 3 times/day; see Table).
Conclusions:GLN and glutathione deficiency contribute to COVID-19 severity, and GLN has salutary biologic actions on reducing lung pathology, mediators of cytokine storm, and myocardial injury in animal models, SCD, and ICU patients. GLN reduces severity in standard risk COVID-19 patientsafterinfection has occurred. These findings, combined with computational prediction of GLN benefit vs. COVID-19, support the hypothesis that PGLG treatmentprior toSARS-CoV-2 infection mayreducethe development of severe COVID-19 in SCD and perhaps other high-risk populations. The data as a whole provides a strong rationale for a controlled clinical trial of PGLG toreducesevere COVID-19 in high-risk SCD patients and improve outcomes if infection occurs.
Cengiz:Biruni University Medical Faculty:Current Employment;Istanbul University-Cerrahpasa Medical Faculty:Ended employment in the past 24 months.Yavuzer:Istanbul University-Cerrahpasa, Cerrahpasa School of Medicine, Department of Internal Medicine, Division of Geriatrics:Current Employment.Yavuzer:Biruni University Medical Faculty:Current Employment;Istanbul University-Cerrahpasa Mediacl Faculty:Ended employment in the past 24 months.Tang:Kaiser Permanente:Current Employment.Ward:Emmaus Medical, Inc.:Current Employment.Goodrow:Emmaus Medical, Inc.:Current Employment;Emmaus Life Sciences Shareholder:Current equity holder in publicly-traded company.Ludlum:Emmaus Life Sciences, Inc.:Consultancy, Current equity holder in publicly-traded company.Stark:Emmaus Life Sciences Shareholder:Current equity holder in publicly-traded company;Emmaus Medical, Inc:Current Employment.Perrine:Cetya Inc.:Membership on an entity's Board of Directors or advisory committees;Phoenicia Bioscience:Current Employment, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees;Phoenicia Therapeutics:Membership on an entity's Board of Directors or advisory committees, Patents & Royalties;Boston University School of Medicine:Current Employment, Patents & Royalties;Viracta Therapeutics:Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal